Leukemia Translocation Panel for Real-Time PCR
Cat Number: LEUKMP-RT24
The Leukemia Translocation Panel is a one-step reverse transcription and qPCR assay designed to quantitatively detect 6 fusion genes commonly found in childhood and adult leukemias.
- One –step cDNA synthesis
- Detects 11 different fusion genes
- 24 tests per kit
- Less than 2-hour turnaround time
- Works with bone marrow or blood
- 2 Reactions per sample
- Low positive control available to assist in assay validation and monitoring
- For research use (RUO) in the U.S
- For in vitro diagnostic use (IVD) in the European Union
Chromosomal translocations are among the most significant genetic alterations in leukemia and serve as key biomarkers for disease classification, prognosis, and the development of targeted therapies. These translocations often result in the fusion of enhancer/promoter regions or coding sequences from two distinct genes, leading to abnormal gene expression, disruption of normal cellular function, or oncogenic activity that drives leukemogenesis. Specific fusion genes are highly prevalent in both pediatric and adult leukemias and are closely associated with disease progression and treatment response.
Accurate detection and identification of fusion gene transcripts in blood or bone marrow samples are essential for diagnosis, prognostic assessment, and treatment stratification in acute lymphoblastic leukemia (ALL), acute promyelocytic leukemia (APL), and acute myeloid leukemia (AML). Beyond initial detection, quantitative molecular monitoring of these fusion transcripts allows for the identification of minimal residual disease (MRD) and early signs of molecular relapse, supporting timely adjustments to therapeutic approaches
The Leukemia Translocation Panel v2 (LEUKMP) is a quantitative real-time PCR-based assay designed for the sensitive detection and monitoring of 11 fusion gene variants associated with six key chromosomal translocations in ALL, AML, and APL. The assay includes validated primer-probe sets and is compatible with major multi-channel real-time PCR platforms, supporting both diagnostic research and longitudinal MRD monitoring.
A low positive control for the Leukemia Translocation Panel is available and sold separately to support assay validation and performance monitoring.
Additionally, one-step RT-PCR kits for individual fusion gene transcripts are available separately for molecular monitoring during treatment.
| Disease | Translocations | Fusion Transcripts |
| ALL | t(1;19) | E2A/PBX1 (e13/e2) |
| t(12;21) | TEL/AML1 (e5/e2) | |
| t(4;11) | MLL/AF4 (e9/e5) | |
| MLL/AF4 (e9/e4) | ||
| APL | t(15;17) | PML/RARα (bcr1) |
| PML/RARα (bcr2) | ||
| PML/RARα (bcr3) | ||
| AML | Inv 16 | CBFB/MYH11 (A type) |
| CBFB/MYH11 (D type) | ||
| CBFB/MYH11 (E type) | ||
| t(8;21) | AML1/ETO (e5/e12) |
For More Information
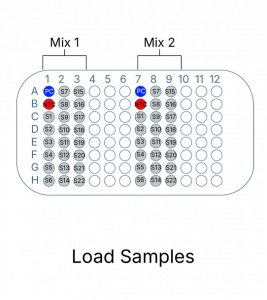
The Leukemia Translocation Panel follows a simple and easy to use process. It can be completed in 2 hours with only 15 minutes of hands-on time
EntroGenʼs leukemia transloacation panel requires a real-time PCR instrument capable of detecting FAM, VIC, ROX, and CY5 fluorescent probes. This test includes reagents required for PCR amplification/detection, as well as validated reaction controls.
The panel does not include reagents or columns for isolation of total RNA from blood or bone marrow.



Click to download lot-specific quality control data for your product:






