Colorectal Cancer Mutation Detection Panel for Real-Time PCR
Cat Number: CRC-RT48
The Colorectal Cancer Mutation Detection Panel is a PCR-based assay that detects somatic mutations in the KRAS, NRAS, BRAF, PIK3CA, and AKT1 genes, which are associated with tumor progression and resistance to anti-EGFR therapies in colorectal cancer.
- 48 tests per kit
- 2-hour turnaround time
- Works with FFPE and fresh frozen samples
- 6 Reactions per sample
- For research use (RUO) in the U.S
- For in vitro diagnostic use (IVD) in the European Union
Mutations in the KRAS, NRAS, BRAF, PIK3CA, and AKT1 genes are frequently found in colorectal cancer and play a significant role in tumor progression and resistance to anti-EGFR therapies such as cetuximab and panitumumab. Identifying these mutations is essential for understanding tumor biology and guiding research on targeted treatment strategies.
The Colorectal Cancer Mutation Detection Panel uses real-time PCR to accurately detect somatic mutations in KRAS, NRAS, BRAF, PIK3CA, and AKT1, even in samples containing a mixture of mutant and wild-type DNA. The assay utilizes allele-specific primers and fluorescent probes for reliable mutation identification and includes an internal control to ensure the integrity of the DNA and reagents.
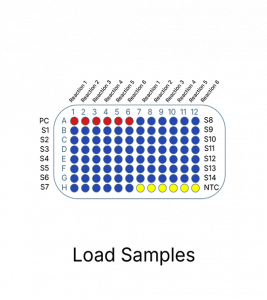
The Colorectal Cancer Mutation Detection Panel is a qPCR assay designed to detect multiple mutations in a total of 6 reactions per sample, providing a comprehensive genetic profile to support colorectal cancer research.
| Gene | Exon | Amino Acid Change | Nucleotide Change | Cosmic ID | Reaction No. |
| KRAS | 2 | G12A | c.35G>C | 522 | 1 |
| G12D | c.35G>A | 521 | 1 | ||
| G12R | c.34G>C | 518 | 1 | ||
| G12C | c.34G>T | 516 | 1 | ||
| G12S | c.34G>A | 517 | 1 | ||
| G12V | c.35G>T | 520 | 1 | ||
| G13D | c.38G>A | 532 | 1 | ||
| 3 | A59T | c.175G>A | 546 | 6 | |
| A59E | c.176C>A | 547 | 6 | ||
| A59G | c.176C>G | 28518 | 6 | ||
| Q61H | c.183A>C | 554 | 4 | ||
| Q61H | c.183A>T | 555 | 4 | ||
| Q61L | c.182A>T | 553 | 4 | ||
| Q61R | c.182A>G | 552 | 4 | ||
| 4 | K117N | c.351A>C | 19940 | 1 | |
| K117N | c.351A>T | 28519 | 1 | ||
| K117R | c.350A>G | 4696722 | 1 | ||
| K117E | c.349A>G | — | 1 | ||
| A146T | c.436G>A | 19404 | 5 | ||
| A146P | c.436G>C | 19905 | 5 | ||
| A146V | c.437C>T | 19900 | 5 | ||
| NRAS | 2 | G12D | c.35G>A | 564 | 2 |
| G12S | c.34G>A | 563 | 2 | ||
| G12C | c.34G>T | 562 | 2 | ||
| G13R | c.37G>C | 569 | 2 | ||
| G13V | c.38G>T | 574 | 2 | ||
| 3 | A59T | c.175G>A | 578 | 6 | |
| A59D | c.176C>A | 253327 | 6 | ||
| Q61K | c.181C>A | 580 | 3 | ||
| Q61L | c.182A>T | 583 | 3 | ||
| Q61R | c.182A>G | 584 | 3 | ||
| Q61H | c.183A>C | 586 | 3 | ||
| Q61H | c.183A>T | 585 | 3 | ||
| 4 | K117R | c.350A>G | — | 2 | |
| A146T | c.436G>A | 27174 | 5 | ||
| BRAF | 15 | V600E | c.1799T>A | 476 | 3 |
| V600E2 | c.1799_1800TG>AA | — | 3 | ||
| V600D | c.1799_1800TG>AT | 477 | 3 | ||
| V600K | c.1798_1799GT>AA | 473 | 3 | ||
| PIK3CA | 9 | E542K | c.1624G>A | 760 | 4 |
| E545K | c.1633G>A | 763 | 4 | ||
| E545Q | c.1633G>C | 27133 | 4 | ||
| 20 | H1047R | c.3140A>G | 775 | 4 | |
| H1047L | c.3140A>T | 776 | 4 | ||
| AKT1 | 4 | E17K | c.49G>A | 33765 | 6 |
Colorectal Cancer Mutation Detection Panel follows a simple and easy to use process. It can be completed in 2 hours and follows a far less complicated process.
EntroGenʼs Colorectal cancer mutation screening panel requires a real-time PCR instrument capable of detecting FAM, ROX, CY5 and VIC fluorescent probes. This test includes reagents required for PCR amplification/detection, as well as validated reaction controls.
Columns and reagents for DNA isolation are not included. Genomic DNA from FFPE tissues can be extracted using EntroGen pureNA® Genomic DNA Isolation Kit.
We suggest using the Internal Quality Control Assay (cat. no. IQCA–RT50) to determine the optimal amount of input DNA prior to running this assay.



Click to download lot-specific quality control data for your product:







