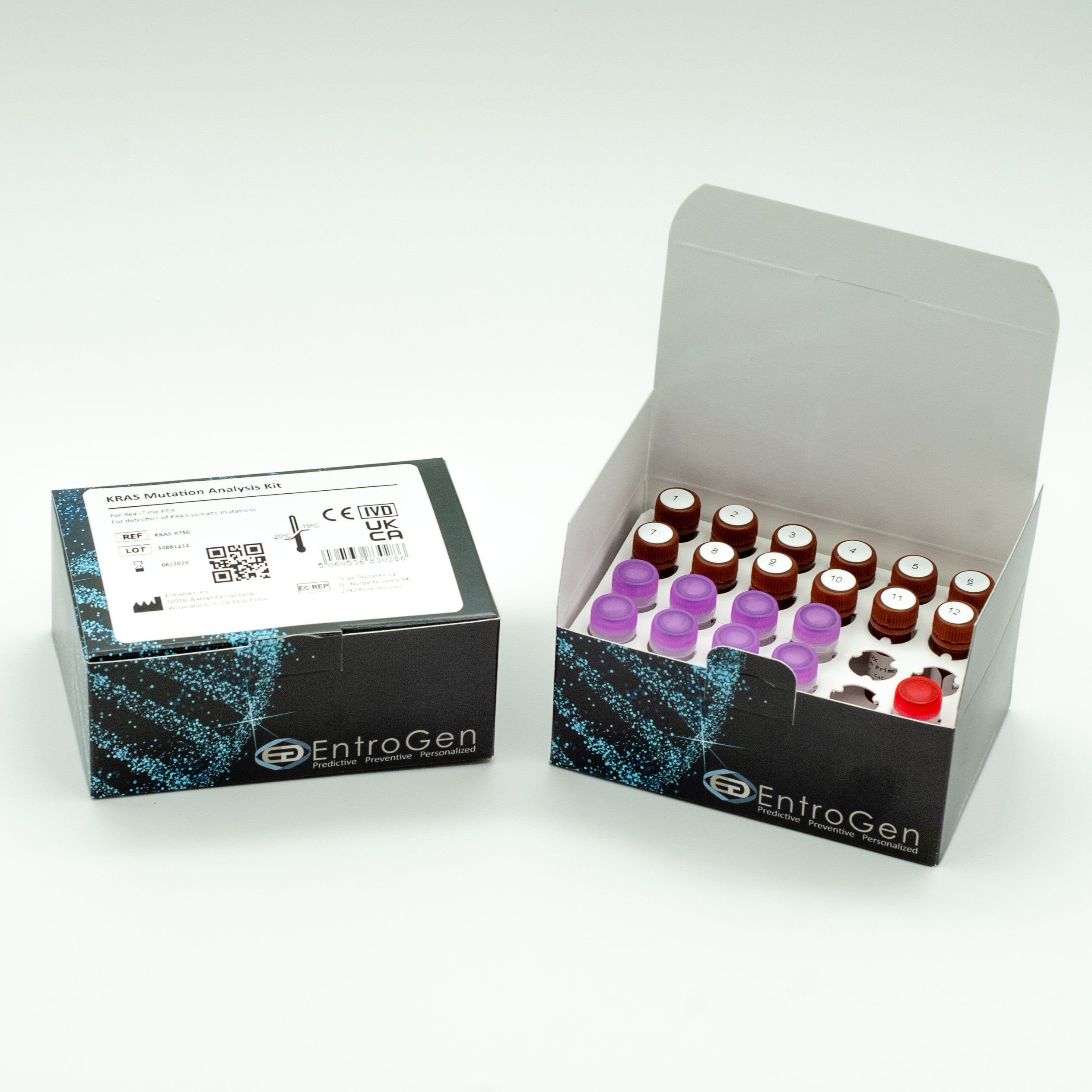
KRAS Mutation Analysis
Cat Number: KRAS-RT50
The KRAS Mutation Analysis Kit is a real-time PCR-based assay that detects somatic mutations in the KRAS gene, which are associated with tumor progression and resistance to EGFR-targeted therapies.
- Below 1% Limit of Detection
- 50 tests per kit
- 2-hour turnaround time
- Works with FFPE and fresh frozen samples
- 12 Reactions per sample
- For research use (RUO) in the U.S
- For in vitro diagnostic use (IVD) in the European Union
The KRAS gene encodes a small GTPase that plays a crucial role in transmitting signals from the epidermal growth factor receptor (EGFR) to downstream pathways involved in cell proliferation and survival. Mutations in KRAS, particularly in codons 12, 13, 61, 117, and 146, are frequently found in cancers such as metastatic colorectal cancer (mCRC), lung adenocarcinoma, and thyroid cancer. Studies have shown that tumors harboring KRAS mutations are less likely to respond to anti-EGFR antibody therapy, making KRAS mutation testing a component of personalized treatment selection.
The KRAS Mutation Analysis Kit is a real-time PCR-based assay designed for the specific and sensitive detection of somatic KRAS mutations. Using allele-specific PCR and fluorescent hydrolysis probes, the assay preferentially amplifies mutant DNA even in samples with a high proportion of wild-type DNA.
An internal control gene ensures sample integrity and amplification efficiency.
The KRAS Mutation Analysis Kit detects the following mutations in KRAS:
| Exon | Mutation | Nucleotide Change | Amino Acid Change | COSMIC ID |
| 2 | G12D | c.35G>A | Glycine (G) to Aspartic acid (D) | 521 |
| G12C | c.34G>T | Glycine (G) to Cysteine (C) | 516 | |
| G12S | c.34G>A | Glycine (G) to Serine (S) | 517 | |
| G12R | c.34G>C | Glycine (G) to Arginine (R) | 518 | |
| G12A | c.35G>C | Glycine (G) to Alanine (A) | 522 | |
| G12V | c.35G>T | Glycine (G) to Valine (V) | 520 | |
| G13D | c.38G>A | Glycine (G) to Aspartic acid (D) | 532 | |
| 3 | Q61H | c.183A>C & c.183A>T | Glutamine (Q) to Histidine (H) | 554 & 555 |
| Q61L | c.182A>T | Glutamine (Q) to Leucine (L) | 553 | |
| Q61R | c.182A>G | Glutamine (Q) to Arginine (R) | 552 | |
| 4 | K117N | c.351A>C & c.351A>T | Lysine (K) to Asparagine (N) | 19940 & 28519 |
| K117R | c.350A>G | Lysine (K) to Arginine (R) | 1178068 | |
| K117E | c.349A>G | Lysine (K) to Glutamic acid (E) | 1360831 | |
| A146T | c.436G>A | Alanine (A) to Threonine (T) | 19404 | |
| A146P | c.436G>C | Alanine (A) to Proline (P) | 19905 | |
| A146V | c.437C>T | Alanine (A) to Valine (V) | 19900 |
For More Information
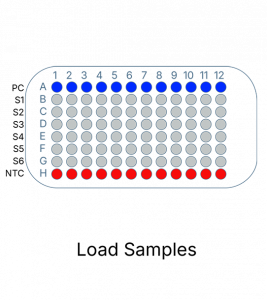
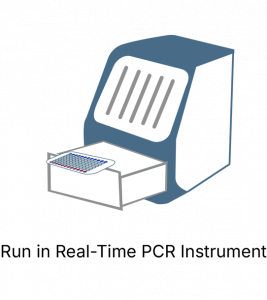
The KRAS Mutation Analysis Kit follows a simple and easy to use process. It can be completed in 2 hours and follows a simple straightforward process.
EntroGenʼs KRAS mutation screening panel requires a real-time PCR instrument capable of detecting VIC and FAM fluorescent probes.
This test includes reagents required for PCR amplification/detection, as well as validated reaction controls.
Columns and reagents for DNA isolation are not included. Genomic DNA from FFPE tissues can be extracted using EntroGen pureNA® Genomic DNA Isolation Kit.
We suggest using the Internal Quality Control Assay (cat. no. IQCA–RT50) to determine the optimal amount of input DNA prior to running this assay.



Click to download lot-specific quality control data for your product: