Microsatellite Instability Kit
Cat Number: MSI-RT24
The MSI Kit is a qPCR-based assay that detects microsatellite instability (MSI) in five mononucleotide repeat regions using high-resolution melting (HRM) analysis to enable rapid, sensitive, and streamlined MSI assessment in colorectal tumor samples.
- 24 tests per kit
- High-resolution melting analysis
- Analysis software included
- 3-hour turnaround time
- Works with FFPE
- 5 Reactions per sample in duplicate
- For research use only (RUO)
Microsatellite instability (MSI) represents a key molecular signature of tumors with defective DNA mismatch repair (dMMR), a condition that leads to the accumulation of insertion or deletion errors within short, repetitive DNA sequences known as microsatellites. MSI is a hallmark of Lynch syndrome (hereditary nonpolyposis colorectal cancer) and is also commonly observed in several solid tumors, including colorectal, endometrial, and gastric cancers. Tumors exhibiting MSI-high (MSI-H) status have distinct biological and clinical characteristics, including increased neoantigen load and enhanced response to immune checkpoint inhibitors, making MSI testing an important tool in cancer research and therapeutic decision-making.
The EntroGen MSI Detection Kit is a qPCR-based assay designed to detect and characterize MSI across five mononucleotide repeat regions: BAT-25 (KIT), BAT-26 (MSH2), NR-21 (SLC7A8), NR-27 (BIRC3), and MONO-27 (MAP4K3). The assay employs high-resolution melting (HRM) analysis to evaluate changes in DNA melting profiles caused by alterations in microsatellite length—an indicator of MSI. By incorporating wildtype sequence blockers, the assay selectively suppresses normal sequence amplification, enhancing sensitivity for detecting MSI-induced shifts within tumor DNA.
This five-reaction assay includes target-specific primer sets and MSI/MSS controls for reliable baseline correction across HRM-enabled thermal cyclers. The method eliminates the need for capillary electrophoresis.
The EntroGen MSI Detection Kit is compatible with a wide range of HRM-enabled real-time PCR instruments. To support streamlined and standardized interpretation, EntroGen provides dedicated analysis software that simplifies data processing and aids in accurate MSI status determination.
|
Reaction No. |
Reporter |
Target |
Gene |
Chromosome |
Position (hg38) |
|
1 |
FAM |
BAT-25 |
KIT |
4 |
54732045 |
|
2 |
BAT-26 |
MSH2 |
2 |
47414420 |
|
|
3 |
NR-21 |
SLC7A8 |
14 |
23183137 |
|
|
4 |
NR-27 |
BIRC3 |
11 |
102322777 |
|
|
5 |
MONO-27 |
MAP4K3 |
2 |
39345921 |
The MSI Detection Kit follows a simple, streamlined process that can be completed in approximately 3 hours, from DNA preparation to result generation.
EntroGenʼs MSI Detection Kit requires a real-time PCR instrument capable of detecting FAM fluorescent probes.
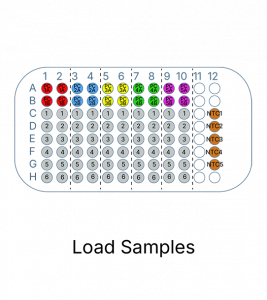
This test includes reagents required for PCR amplification/detection, as well as validated reaction controls. A single 96-well plate can accommodate up to six unknown samples in duplicate along with the required controls.
Columns and reagents for DNA isolation are not included. Genomic DNA from FFPE tissues can be extracted using EntroGen pureNA® Genomic DNA Isolation Kit.
To support data interpretation, EntroGen provides dedicated MSI Analysis Software that simplifies melting curve analysis and aids in accurate determination of MSI status.