Passion For Quality
Quality Policy Statement
EntroGen, Inc. undertakes to comply with applicable regulatory requirements on its products and commits to provide customers with quality products and supports that meet or exceed their requirements and expectations. By maintaining and continuously improving business processes we will enhance the value of our company to our customers.
More than
Kits Manufactured
More than
Patient Samples Tested
More than
Adverse Events Reported
More than
%
QC Release Compliance
Less than
Hour technical support inquiry turnaround time
Products shipped to
Countries around the world
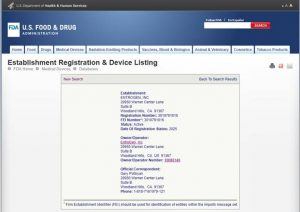
FDA Approved Manufacturing Facility / cGMP
FDA Establishment Registration Number: 3010791816
Owner/Operator Number: 10088140
ISO 13485 Certified Quality Management System
EntroGen Newsletter
Receive quarterly updates on EntroGen news and products